
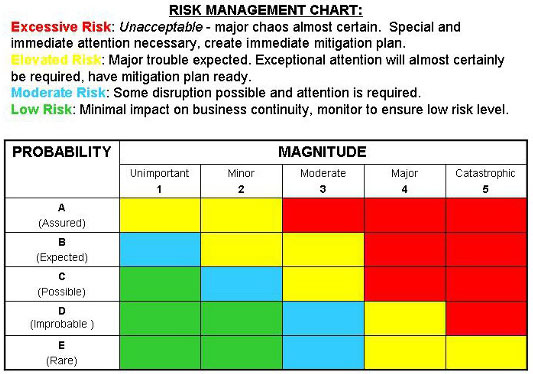
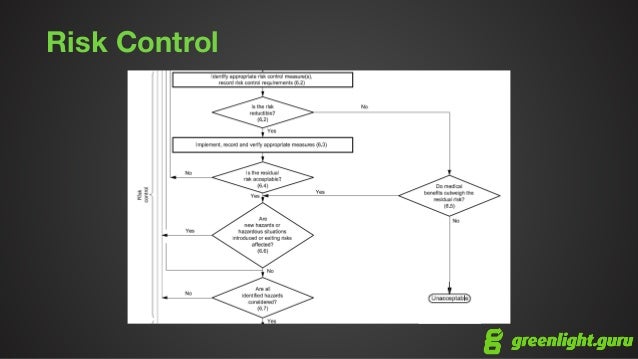
It’s important that your most knowledgeable people, in terms of the design, manufacture, distribution and use of your device, are involved with the risk identification and evaluation process. Clauses 7.4 and 8 of ISO 14971:2019 emphasize the need to evaluate residual risk, while Clause 3.15 provides guidance on how to minimize known and foreseeable risk - your evaluation process should include definitions for both of these types of risk. You might wonder, how far do you have to go with your risk management using these tools? Must everything be considered for a device to be safe and effective?Ī good rule of thumb is to focus on risks that are within your control.
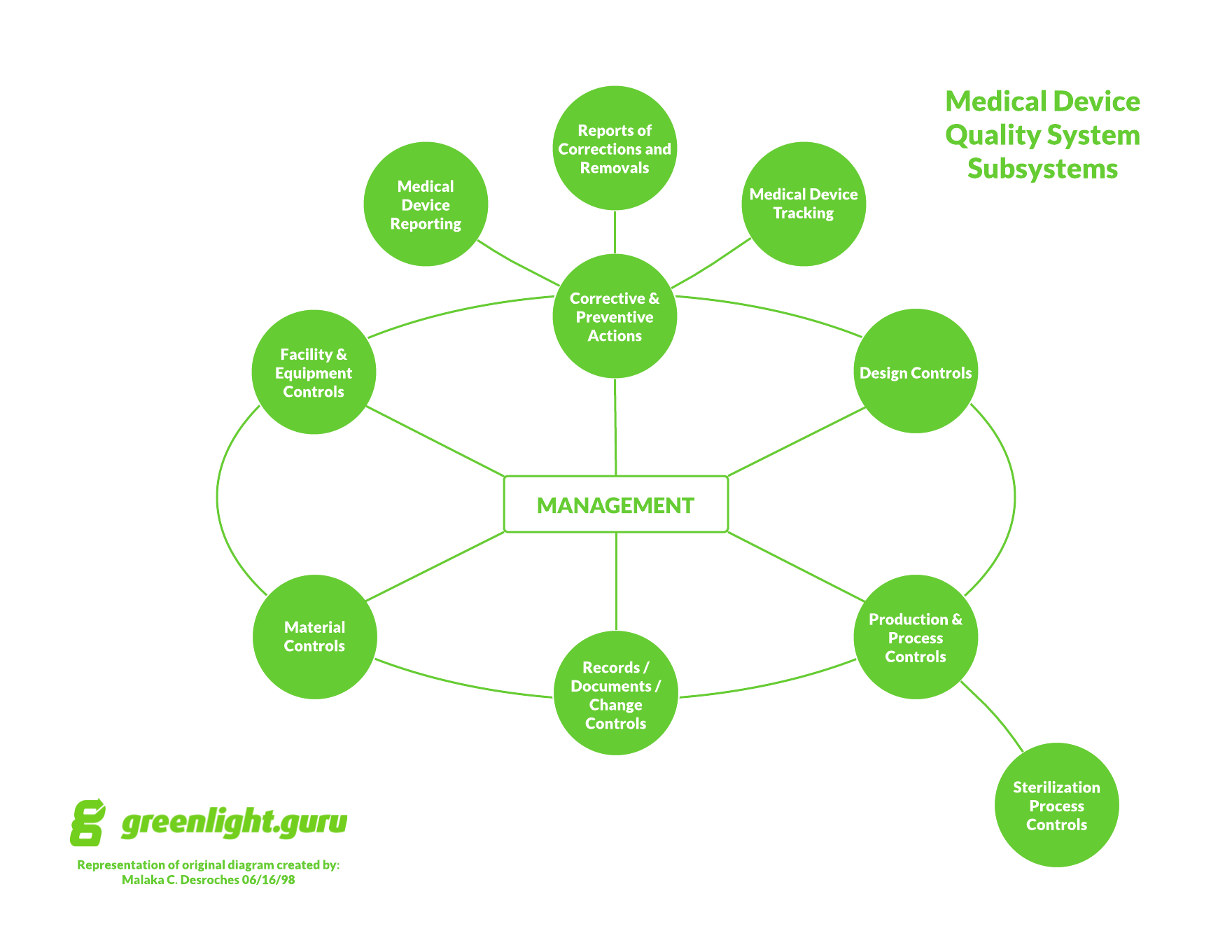
Your risk management file will prove to be an instrumental asset throughout the life cycle of your medical device. Regardless of which risk management tools you’re using, you should always document your processes, any identified risks, and rationale scrupulously in a living risk management file. The purpose of risk management tools is to help manufacturers identify and analyze risks before then taking action to prevent, mitigate, or reduce those identified risks to acceptable levels.
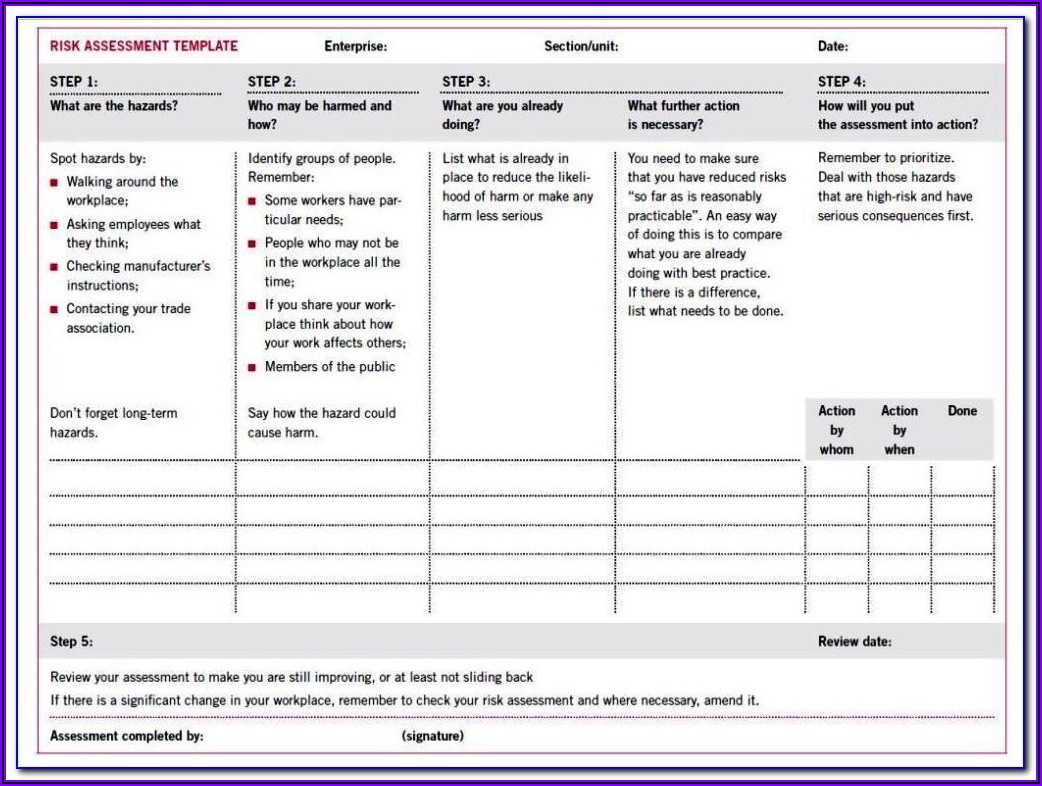
Risk management tools in the medical device industry This article will take you through some of the most commonly used tools and methods, so you can make an informed decision the next time you’re considering which one to use.įREE RESOURCE: Click here to download our previously confidential Risk Management Plan Template. There are a handful of risk management tools used in the medical device industry to help manufacturers manage risk. Especially when everything comes back to the seemingly subjective question of: A m I effectively managing risk?Ī key part of answering “yes” to this question, and doing so with confidence, involves following a planned, disciplined risk management process in which risk is thoroughly evaluated, controlled, and reduced to acceptable levels. There can be so many parts and pieces involved throughout the life cycle of a medical device that sometimes risk management activities can appear quite onerous. The role of risk management for medical devices is not just a regulatory expectation, it’s a critical part of designing, developing, and manufacturing safe and effective devices for patients.


 0 kommentar(er)
0 kommentar(er)
